Electron Configurations Of Silicon And Germanium Atoms
![]()
REVIEW OF SEMICONDUCTOR PHYSICS
- The electronics subject begins from the concepts of behaviour of charge carriers in electron devices and Integrated Circuits (ICs) under influence of electric fields.
- A model of an atom is shown in Fig. 2.1. The aspect of electron motion is analogous to the planetary motion in which the planets rotate round the sun. On similar lines, electrons move in closed stationary orbits around the positive nucleus in an atom.
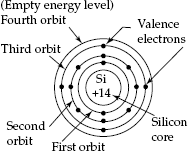
Fig. 2.1 Electron configuration of silicon atom
2.1.1 Electron Configurations of Silicon and Germanium Atoms
- The shell structure and states occupied by electrons depend on the valence of material and its atomic number Z. Silicon and Germanium semiconductor materials are used for the manufacture of semiconductor devices.
The distribution of electrons in the various orbits for Silicon and Germanium atoms is shown in Table 2.1 and in Figs. 2.1 and 2.3.
Table 2.1
| Element | Atomic number (Z) | Configuration |
|---|---|---|
| Silicon (Si) | 14 | 1s2 2s2 2p6 3s2 3p2 |
| Germanium (Ge) | 32 | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 |
Electron configuration of Silicon atom (Fig. 2.1)
- The atomic number of Silicon atom is Z = 14. It contains 14 positive charges in the nucleus and 14 electrons that move about the nucleus in closed stationary orbits. The orbits are assumed to be concentric circles. Thus, each atom is electrically neutral (Zero charge for the atom as a whole). Hence, the Silicon material is an ‘Electrically Neutral material’.
- The planetary model for the atom is considered only from the classical model. Each ‘Silicon atom’ has its electrons arranged in groups of energy levels or shells as the following:
- First orbit, the inner most energy level has 2 electrons (completely filled).
- Second orbit has 8 electrons (completely filled).
- Third orbit has the balance of 4 electrons (partially filled).
- Energy levels starting from the fourth level are empty energy levels.
- This last partially filled shell (third orbit) is called valence shell.
- The 4 electrons in the third orbit (shell) are known as valence electrons.
- Valence electrons are responsible for the chemical and electrical properties of the material.
- Electrons extracted from valence shell and not subject to force of attraction of nucleus on them are called free electrons.
Silicon atom representation as a tetravalent material is shown in Fig. 2.2 as a basis to understand the concept of covalent bond formation etc. Silicon semiconductor, as a ‘Tetravalent’ material, has ‘four valence electrons’. The force of attraction between the nucleus (core) and the electron inside the atom is given by
![]()
where electronic charge q is in coulombs, r is the separation distance between electrons and nucleus (in an atom) in metres, the force F is in Newtons, ɛ0 is the permittivity of free space in farads/metre, and permittivity of the free space ɛ0 = 8.849 × 10–12 farads/metre.
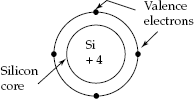
Fig. 2.2 Representation of silicon atom with its valence electrons
This force of attraction F between the nucleus and the electron is counter balanced by
![]()
where m is the electronic mass, m = 9.109 × 10–31 kg, v is the speed of the electron in the orbit, acceleleration a = v2/r and is directed towards the nucleus.

![]()
The potential energy PE of the electron at a distance ‘r’ from the nucleus ![]() .
.
According to the conversation of energy, energy associated with the electrons W = Kinetic energy + Potential energy:
![]()
where the energy W is in joules.
Substituting the value ![]() from Eq. (2.2A) into Eq. (2.3), we get
from Eq. (2.2A) into Eq. (2.3), we get
![]()
Equation (2.4) shows the relation between the radius r (distance of electron in the circular orbit from the nucleus) and the energy W of the electrons. It also shows that the energy of the electron becomes less (i.e., more negative) as it approaches closer to the nucleus. The relation is given by Eq. (2.4A):
![]()
where n = 1, 2, 3 and so on.