Atomic Structure And Electric Charge
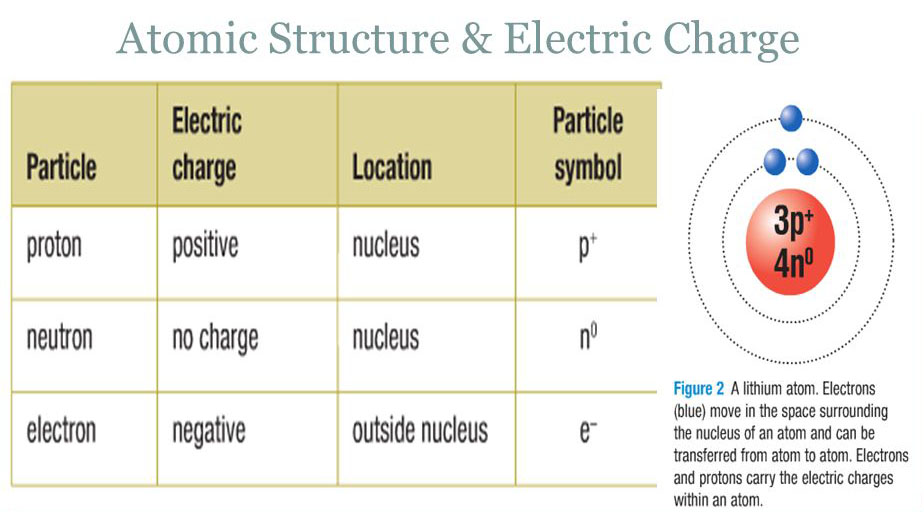
Several theories have been developed to explain the nature of electricity. The modern electron theory of matter, propounded by scientists Sir Earnest Rutherford and Niel Bohr considers every matter as electrical in nature. According to this atomic theory, every element is made up of atoms which are neutral in nature. The atom contains particles of electricity called electrons and protons. The number of electrons in an atom is equal to the number of protons.
The nucleus of an atom contains protons and neutrons. The neutrons carry no charge. The protons carry positive charge. The electrons revolve round the nucleus in elliptical orbits like the planets around the sun. The electrons carry negative charge. Since there are equal number of protons and electrons in an atom, an atom is basically neutral in nature.
If from a body consisting of neutral atoms, some electrons are removed, there will be a deficit of electrons in the body, and the body will attain positive charge. If neutral atoms of a body are supplied some extra electrons, the body will attain negative charge. Thus, we can say that the deficit or excess of electrons in a body is called charge.
Charge of an electron is very small. Coulomb is the unit of charge. The charge of an electron is only 1.602 × 10–19 Coulomb (C). Thus, we can say that the number of electrons per Coulomb is the reciprocal of 1.602 × 10–19 which equals approx. 6.28 × 1018 electrons. Therefore, charge of 6.28 × 1018 electrons is equal to 1C. When we say that a body has a positive charge of 1C, it is understood that the body has a deficit of 6.28 × 1018 electrons.
Any charge is an example of static electricity because the electrons or protons are not in motion. You must have seen the effect of charged particles when you comb your hair with a plastic comb, the comb attracts some of your hair. The work of combing causes friction, producing charge of extra electrons and excess protons causing attraction.
Charge in motion is called electric current. Any charge has the potential of doing work, i.e., of moving another charge either by attraction or by repulsion. A charge is the result of separating electrons and protons. The charge of electrons or protons has potential because it likes to return back the work that was done to produce it.